
Watch the video and see if you missed any steps or information. Valency can be defined as the capacity of an atom or radical to combine with other atoms or radicals.From the valence-state ionization potentials, it is concluded that the. Try to draw the CO Lewis structure before watching the video. Electronegativity values (as defined by Mulliken) are obtained for Ni and Cu.

The Lewis structure for CO has 10 valence electrons.įor the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. After determining how many valence electrons there are in CO, place them around the central atom to complete the octets. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell with a noble gas electron configuration ending in ns2np6.

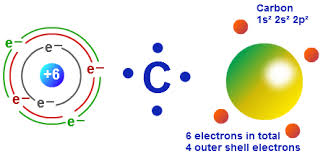
While helium normally has a 0 valence, it seems to have a weak tendency to. The basic metals are similar to transition metals but tend to be softer and to hint at nonmetallic properties. Description The combining capacity, or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. Using liquid helium, Kurti and co-workers and others, have succeeded in. Two electrons in the valence shell Readily form divalent cations Low electron affinity Low electronegativity Transition Metals The lanthanides (rare earth) and actinides are also transition metals. Video: Drawing the Lewis Structure for COįor the CO Lewis structure, calculate the total number of valence electrons for the CO molecule. In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. valence electrons given by carbon atoms 4 1 4 valence electrons given by oxygen atoms 6 1 6 Total valence electrons 10 Total valence electrons pairs Total valance electrons pairs bonds + bonds + lone pairs at valence shells Total electron pairs are determined by dividing the number total valence electrons by two.


 0 kommentar(er)
0 kommentar(er)
